
GrayMatter Partners with Tailwind to Transform Industrial Intelligence
May 2, 2024
TechHub: Live from Automate 2024
May 16, 2024
GrayMatter Partners with Tailwind to Transform Industrial Intelligence
May 2, 2024
TechHub: Live from Automate 2024
May 16, 2024ProjectX: Cutting-Edge Serialization Advances Packaging Compliance for Pharmaceutical Companies
Advancing Packaging Compliance for Pharmaceutical Companies
GrayMatter’s engineering team completed serialization projects at three companies that provide pharmaceutical packaging services.
Serialization presents unique challenges because of the regulatory requirements for product traceability and anti-counterfeiting measures in the pharmaceutical industry.
The team’s work falls under GrayMatter’s Automation & Controls Offering, which includes PLC programming, SCADA/HMI design, machine control, control logic testing, and many more as described in our Automation & Controls offering.
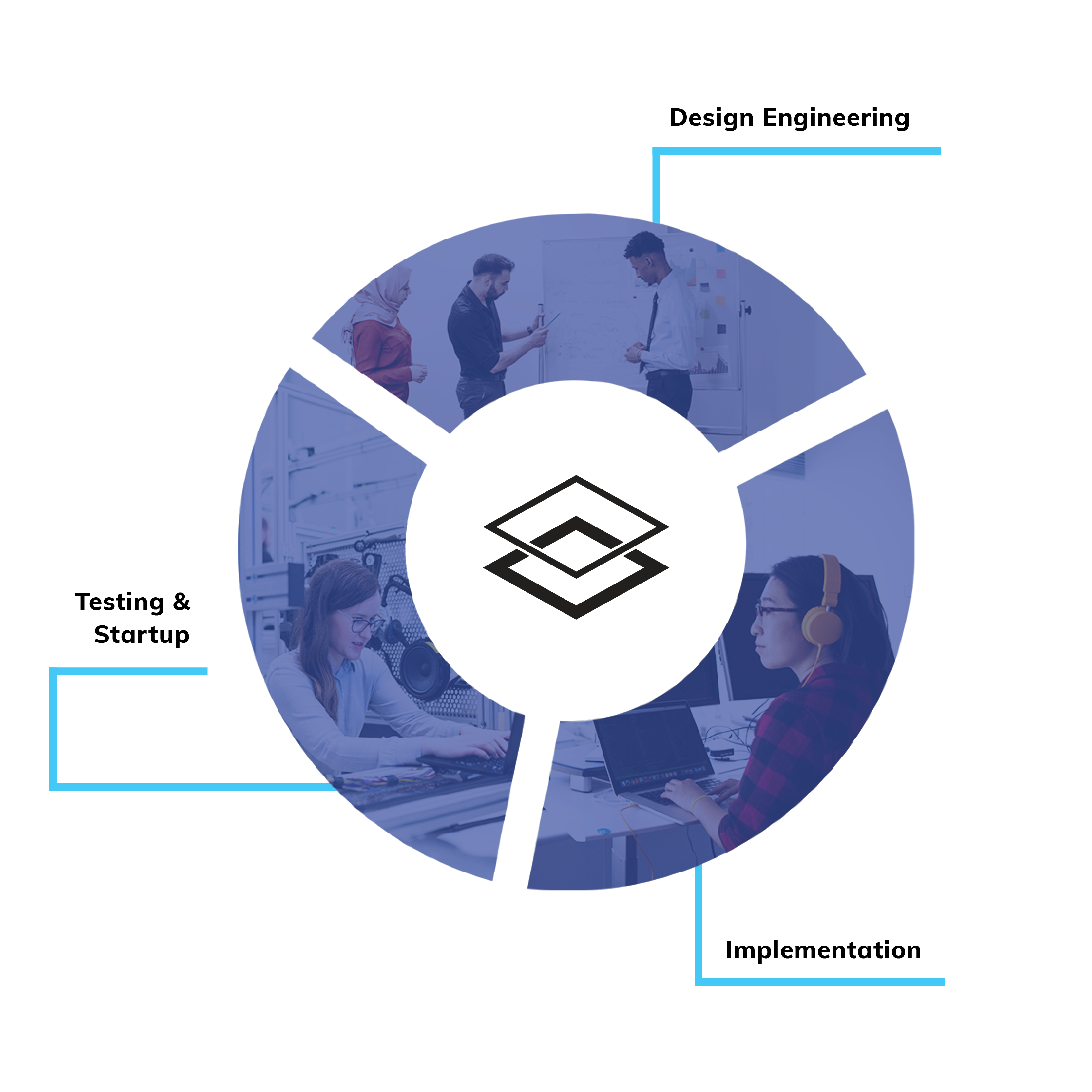
WHAT IS SERIALIZATION?
Serialization refers to the process of assigning a unique identifier to items as they go through a production or packaging line. The identifier can be, for example, a serial number, barcode, QR code or RFID tag. It allows items to be tracked through a lifecycle.
The Problem
The primary challenge is to meet FDA and international regulatory requirements for serialization, ensuring that clients can track and trace products across various markets with different standards.
An additional objective is to meet special formatting requirements for reporting and graphics that are unique to individual customers.
Serialization demands a complex data handshake and association between different packaging levels, calling for sophisticated technology and a skilled team.
Serialization demands a complex data handshake and association between different packaging levels, calling for sophisticated technology and a skilled team.
The Solution(s)
GrayMatter’s team includes serialization system experts and machine control/integration specialists. The solutions are multifaceted:
The Results
Regulatory Compliance
The solutions allow the three clients to meet FDA and international serialization regulations, enabling them to trace products back to the manufacturing site and verify authenticity.
With advanced hardware and software in place, the engineering team can print and inspect codes that can be traced at all packaging levels. This capability significantly increases the safety and tracking of products.
Increased Efficiency & Support
Deploying scanners, printers, and manual/auto-focus cameras in the inspection processes leads to faster and more efficient packaging inspections.
The team maintains a strong support system and provides on-site assistance and insights into current technologies, such as new camera systems and scanners.
The project is a complex initiative that combines technological upgrades, expert knowledge and a deep understanding of regulatory requirements.
The project not only meets current compliance needs but also paves the way for future advancements in product tracking and anti-counterfeiting measures. The team's dedication to customization, continuous improvement and co-innovation with clients plays a critical role in the success of the projects.
With advanced hardware and software in place, the engineering team can print and inspect codes that can be traced at all packaging levels. This capability significantly increases the safety and tracking of products.
Start a Project
Talk to Our Team of Experts
Monday - Friday EST
08:00 AM - 5:00 PM
